Abstract
MBD4, a gene encoding a DNA glycosylase functioning in base excision repair, has recently been described in association with predisposition to early onset acute myeloid leukemia (AML), uveal melanoma and colorectal polyposis. MBD4 is essential for guarding against methylation damage due to spontaneous deamination of 5-methylcytosine and biallelic loss of MBD4 allows CG>TG mutations to accumulate in the genome predisposing to AML (Sanders et al. Blood 2018). Further understanding of the full phenotypic spectrum and mechanisms leading to cancer evolution for this predisposition syndrome is critical for informing early recognition, diagnosis, management, and treatment for these individuals and their at-risk family members with the ultimate goal of improving outcomes. Here we report novel germline mutations in MBD4, expand the spectrum of cancers and describe the mutational signatures associated with this cancer predisposition syndrome.
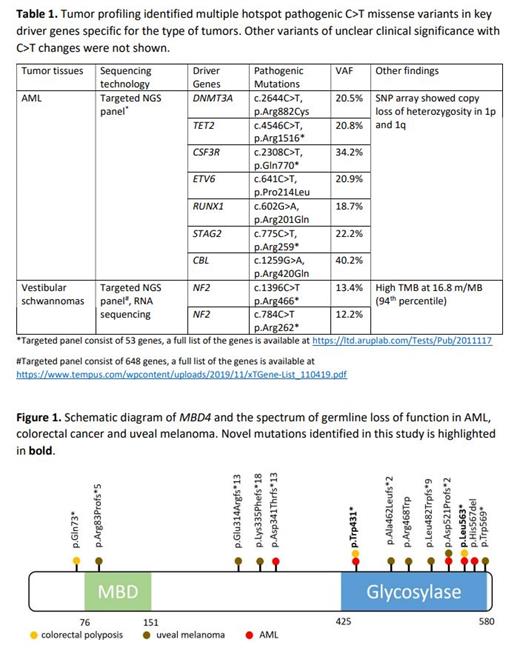
Two siblings (brother and sister) from a non-consanguineous family with strong family history of cancers were diagnosed with early-onset AML and colorectal polyposis in their 30s. Prior to the diagnosis of AML, the brother was diagnosed with colorectal cancer and lymphoma at 24 and 28 years old respectively. He was diagnosed with AML at 30 years old and underwent allogenic hematopoietic stem cell transplant (HSCT) using her sister as donor but subsequently passed away following relapse of his AML. The sister was diagnosed with colorectal polyposis at 33 years old and underwent total colectomy due to numerous (>70) hyperplastic and adenomatous colorectal polyps. At the age of 35 years old she was diagnosed with AML after presenting with progressive peripheral cytopenias, lymphadenopathy and splenomegaly. Her AML was characterized by normal karyotype and absent of NPM1, CEBPA and FLT3 aberrations. Further molecular profiling using targeted myeloid NGS panel identified multiple C>T missense mutations including hotspot DNMT3A mutation c.2644C>T, p.R882C (Table 1). SNP microarray revealed balanced genome but identified copy loss of heterozygosity in chromosome 1p and 1q. She received two cycles of remission induction chemotherapy (7+3 regimen) and achieved remission prior to allogenic HSCT using matched unrelated donor. Her post-transplant course was complicated by chronic graft versus host disease treated with tacrolimus. She currently remains in remission now six years post-transplant.
She later developed papillary thyroid carcinoma at the age of 44 for which she underwent total thyroidectomy. Shortly after, she was evaluated with brain MRI due to sensorineural hearing loss, which identified bilateral vestibular schwannoma. Her right schwannoma was resected and the smaller, left schwannoma is being monitored. Tumor profiling on tissue from the right schwannoma detected two C>T truncating mutations in NF2 as well as multiple other C>T missense variants resulting in high tumor mutational burden calculated at 16.8 mutations/MB (Table 1). Tumor profiling performed on tissue from her thyroid carcinoma is pending.
Due to concern for germline cancer predisposition syndrome, whole exome sequencing was performed and identified two germline nonsense variants in MBD4 c.1291 C>T, p.Arg431* and c.1688 T>A, p.Leu563* in trans configuration. These truncating variants are located in the glycosylase domain of MBD4 and predicted to abolish the catalytic function of the gene (Figure 1). Both truncating variants (Arg431* and Leu563*) are present in gnomAD with very low allele frequency of 3.18 X 10 -5 and 7.04 X 10 -5 respectively. No additional germline mutations were identified.
This case further validates the role of MBD4 as germline predisposition to myeloid malignancies characterized by hyper-mutated genomic signatures. Additionally we expanded the spectrum of cancers associated with this novel cancer predisposition syndrome to include papillary thyroid carcinoma and vestibular schwannomas. Genomic profiling of the normal and affected tissue identified germline bi-allelic loss of function mutation in MBD4 as initiator of methylation defect in key driver genes in tissue specific manner leading to carcinogenesis. This conserved path to mutagenesis is unique to this cancer predisposition syndrome and further biological studies are needed to fully understand the spectrum of cancers associated with this syndrome.
Majhail: Anthem, Inc: Consultancy; Incyte Corporation: Consultancy.